Cold Ethanol Extraction: How it Works
What is Cold Ethanol Extraction?
Ethanol exists as a liquid at room temperature and is best known as the intoxicating chemical in all alcoholic beverages, but that’s not all that its useful for. In modern times ethanol has seen a rise in use as a solvent in the manufacture of cannabis and hemp derivatives. In fact, in this burgeoning new industry, ethanol has become one of the most popular solvents used in the large-scale extraction of the cannabinoids such as THC and CBD.
Is ‘Cold’ Ethanol Extraction the same as ‘Cryogenic’ Ethanol Extraction?
In the cannabis extraction industry, the term “cryogenic ethanol extraction” is often used to describe what is simply—and technically—cold ethanol extraction. The term has widespread colloquial use in the cannabis industry but to call cold extraction ‘cryogenic’ is scientifically incorrect.
When extracting cannabinoids using ethanol, temperature is a critical factor. Too warm and you risk extracting undesirable and unwanted compounds. Too cold and your efficiency and profit margin may be significantly reduced. Therefore, cold ethanol extraction is often the preferable method with an optimal temperature range ideally between -30˚C (-22˚F) and -40˚C (-40˚F).
(*Note, ethanol extraction of cannabinoids may also be performed at ambient or room temperature, but additional post-processing steps may need to be performed to ensure a positive result incurring cost. Learn more about the differences between cold vs. room temperature extraction.)
The term cryogenic extraction refers to any extraction performed between -153°C (-243.4°F) and absolute zero or -273°C (-523.4°F), which is much too cold for ethanol extraction. In fact, ethanol’s freezing point is at -114.1°C (-173.5°F) rendering it useless as an extraction solvent at these temperatures.
The definition of the term “cryogenic” is defined as the production and behavior of materials at extremely low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cryogenic” by accepting a threshold of 120 K (or –153 °C) to distinguish these terms from the conventional refrigeration. This is a logical dividing line, since the normal boiling points of the so-called permanent gases (such as helium, hydrogen, neon, nitrogen, oxygen, and normal air) lie below −120 °C while the Freon refrigerants, hydrocarbons, and other common refrigerants have boiling points above −120 °C. The U.S. National Institute of Standards and Technology considers the field of cryogenics as that involving temperatures below −180 °C (93 K; −292 °F).
− Source: Wikipedia “Cryogenics”
Why Choose Cold Ethanol for Cannabis Extraction?
Ethanol makes for an ideal solvent for botanical oil extraction for many reasons, but the most influential reasons to choose cold ethanol as a solvent when it involves cannabis or hemp extraction are:
- Its chemical structure lends it the ability to extract or dissolve most polar as well as non-polar molecules. Regarding cannabis, this makes ideal for ‘full spectrum’ products. This capability may also be manipulated by solvent temperature.
- Because it exists as a non-viscous liquid at ambient temperatures and pressures (unlike other popular solvents such as CO2 and hydrocarbons) it extracts the molecules very quickly and easily. And much faster process times when compared to hydrocarbons and CO2, for example.
The basics of the cold ethanol extraction process are quite simple to understand and very similar to the way in which other solvents are utilized as well. Almost all solvent extractions of cannabis or hemp consist of three primary steps, usually accompanied by a few other minor processes depending on your SOP and desired end-product goal: Extraction, Evaporation, and Distillation.
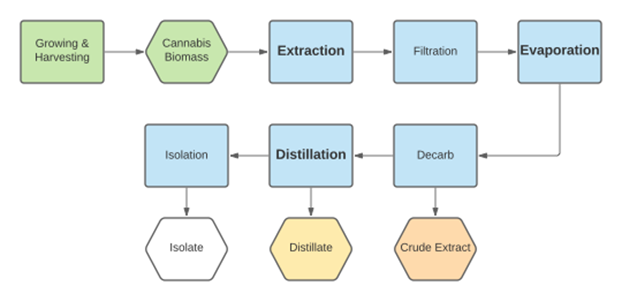
The basics of the cold ethanol extraction process are quite simple to understand and very similar to the way in which other solvents are utilized as well. Almost all solvent extractions of cannabis or hemp consist of three primary steps, usually accompanied by a few other minor processes depending on your SOP and desired end-product goal: Extraction, Evaporation, and Distillation.
In the second step of Evaporation, we take the resulting solution of the first step (a combination of solvent and our target compounds THC / CBD etc.) and then apply vacuum and heat. This allows the solvent to boil off, leaving behind desired compounds in their crude form, this is also known as crude oil—averaging between 40-60% cannabinoid potency.
Finally, the third step of Distillation is a process very similar to Evaporation in which we apply even deeper vacuum and add more heat. However, this time we are attempting to target and ‘boil’ the cannabinoids themselves (in contrast to us targeting the solvent during evaporation) to further refine and separate them. This stage usually results in a distillate that is 85-95% potent.
The Advantages of Cold Ethanol Extraction
Now that we understand the basics of an ethanol extraction process, we can now dive into the cold ethanol extraction process. The process itself is the same save for two significant differences:
- Obviously, as the name suggests the biggest difference is that we will cool the ethanol down to approximately -40˚ (Celsius or Fahrenheit, it doesn’t matter because the two scales are identical at this temperature) prior to extraction and then ensure it does not drop below -30˚C during the extraction process.
- The real advantage comes after the extraction, when your filtration steps become negligible other than a simple particulate filtration.
It should be of note that to properly maintain cold temperatures during extraction it is advised that operators not only chill their ethanol down to -40˚C prior to extraction but also—and just as importantly—they should pre-freeze the biomass in preparation for extraction and jacket their extraction vessel with a chiller.
Both lipids and chlorophyl are chemicals you decidedly do not want to be ingesting via inhalation. They also significantly decrease the overall quality (especially visually) of your end distillate oil. However, they can both easily be eliminated via the cold ethanol extraction process.
Why is this?
The simple answer is that both chlorophyl and lipids generally remain as frozen solids trapped within the plant during extraction in colder conditions. The other factor at play is that ethanol’s ability to dissolve polar compounds is greatly diminished when it is cold, so it will preferentially extract the non-polar molecules first.
So, the key the advantages of cold ethanol extraction are primarily:
- Less labor and time involved which delivers a significant cost reduction due to more efficient processes.
- Filtration steps are significantly reduced to a single filtration step aimed at simple particulates, requiring less equipment, time and labor.
- Quality of your end distillate is increased significantly. Both visually and in its cannabinoid potency.
The Disadvantages of Cold Ethanol Extraction
There are also a few disadvantages to the process of cold ethanol extraction when compared to the standard room or ambient temperature process.
First is the added cost (in most cases significant) of energy and the purchase of cold ethanol extraction equipment to cool the ethanol, store frozen biomass, and jacket your extraction vessel with a decent chiller. While the benefits of the process are significant, so is the upfront cost of purchasing cold ethanol extraction equipment along with the increase in overall energy consumption.
Secondly, because we are attempting to allow the ethanol to dissolve certain components and to not dissolve others, you’ll need to monitor the extraction process closely. You may even want to end the extraction a bit early so the ethanol doesn’t grab any of the undesirable compounds you’re avoiding making sure the ethanol doesn’t warm up. This translates to an overall decrease in processing yields in comparison to the standard room temperature process.
Because of the inherent advantages and disadvantages of both processes neither one is the correct way to process and which one you choose will depend on your budget, staff expertise, and desired end-product.
What Equipment do you need for Cold Ethanol Extraction?
To extract cannabinoids using cold ethanol extraction you’ll need a suite of ethanol extraction equipment.
- Chilling: Pre-chill ethanol solvent using the DC-40 Direct Chiller to as low as -40c to reduce post-extraction steps.
- Extraction: Soak cannabis biomass in chilled ethanol solvent to extract desired compounds via CUP Series closed-loop mechanical centrifugation.
- Particulate Filtration: Remove any suspended particulates/adsorbents with an Ethanol Filtration Skid.
- Solvent Evaporation: Remove ethanol from plant crude oil using the Falling Film Evaporator (FFE).
- Decarboxylation: Activate acidic version of cannabinoids (e.g., CBDa, THCa, etc.) to produce CBD, THC, etc. (depending on your biomass) by removing carboxyl group of molecules.
- Distillation: Separate cannabinoid molecules from crude oil utilizing Rolled Film (Short Path) Distillation (RFE).
- HPLC: Test distillate to measure concentration of cannabinoid molecules.
Note, the list above considers some of the largest equipment purchases necessary for cold ethanol extraction, but it is not exhaustive. There are other ancillary pieces of cold extraction equipment that would be needed for a fully integrated and efficiently functioning ethanol extraction lab, you can read about those here.